For those of us in printing, especially package printing, understanding and keeping up with government labeling regulations can be a thorn in the side. Now don’t roll your eyes or start huffing at the thought just yet. If we choose to look at the glass as half full, we can embrace these changes as “opportunities”! On the one hand, a steady feed of changes or updates to regulations for things like food and drug packaging means job security and another “opportunity” to make a little money for printers and prepress shops. For the CPCs (Consumer Product Companies), it opens the door (another way to say “opportunity”) for consideration of other changes as well, perhaps a chance to give the graphics a little face lift. Right! …But I am guessing these are not your initial sentiments when you are generating a barcode or hear about a new set of requirements for nutritional panels. Frustration, confusion and fear, mixed with a little irritation are probably more common reactions.
What makes us quake a little? I think it is the uncertainty of a system filled with hard to discern rules and yet the potential to face stiff penalties when not followed correctly. Some specifications are general while others are very specific and there are rules that apply to all aspects of the label or package to include font sizes, product identification, manufacturer information, quantity information, where art (or nonessential material) is NOT allowed, food additives and ingredients, food allergens, nutritional information, barcodes and warning statements. Now the regulations I am discussing within this article are more specifically related to the graphics, for things like font specifications, barcodes, ingredients panels and nutritional panels.
Food and drug labeling requirements come from and are governed by a variety of sources, most notably the FDA, USDA, EPA and GS1. While the CPCs are, in general, the most well versed and equipped to ensure the product meets these labeling requirements, it is important that each member in the supply chain does their part to help ensure compliance before the product ends up on the shelf. I expect most CPCs are armed with a team of individuals that keep up with these regulatory sources and determine when and how a label or package is to be updated. As a prepress provider though, you can help establish your relevance as the CPC’s supplier if you have check points built into your process to ensure you are following the latest rules and employing the current formats.

The New and Improved Nutritional Panel
Take for example the FDA’s notification in 2014 about upcoming changes to the nutritional panel. A little history on the matter may be helpful. In the early 1860’s our government initiated the Bureau of Chemistry and the Department of Agriculture which eventually became what we know today as the Food and Drug Administration (FDA, effective 1930). However, it wasn’t until 1990 that the Nutrition Labeling and Education Act (NLEA) was passed, at which point the FDA really began to standardize health claims to include the ingredient information, serving sizes and terminology indicating a product as “low fat” or similarly. By 1993 the first version of a nutritional table with per serving information was finalized and has for the most part remained unchanged, except for the addition of trans fat information in 2006. Fast forward…to 20 May 2016 - After a few years of deliberation, the FDA has finally released the new and improved nutritional table format aimed to make it easier to read and understand (see image 1). Besides the obvious fresh look and design, these updates incorporate a greater understanding of nutrition science and updated serving size requirements to better reflect how we eat today and more realistic serving size specifications. All in an effort to make the label and information within it a more valuable tool for consumers to correlate to health risks like obesity, high blood pressure and cardiovascular diseases.
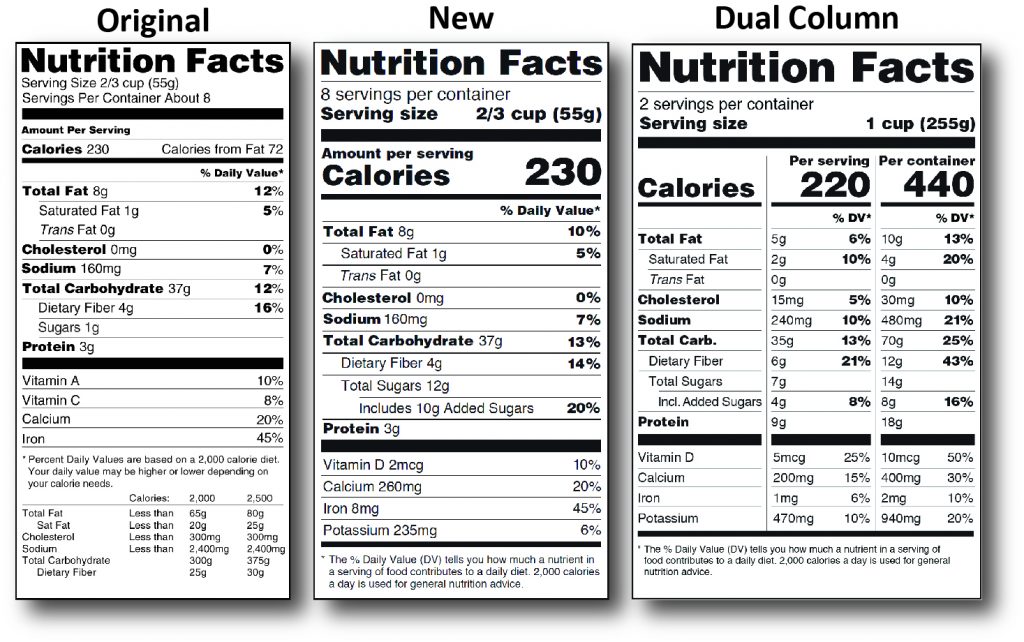
Image 1: Examples from FDA website - Original vs. New vs. Dual Column Panels
Highlighting the Differences (see image 2)
- Parts of the label, such as calories, servings per container and serving size are now more pronounced.
- % Daily Values for nutrients are based on updated scientific information to better guide consumers to not exceed consumtion levels for these nutrients.
- You will also notice some new information, like Added Sugars, while other information has been removed, such as Calories from Fat.
- The list of nutrients required will now also include Vitamin D and potassium. Vitamins A & C are no longer required, but are permitted. Calcium and Iron will continue to be required. Also the actual amounts for these nutrients must be declared in addition to % Daily Value.
- The footnote has changed to more clearly state what is meant by the % Daily Value.
- Serving sizes have also changed to more accurately reflect a true serving size based on today’s standards of what people actually consume versus what they “should” consume. (see image 3)
- The serving size will also better reflect the package size to minimize confusion. For packages that are not meant to be a single serving but could be consumed this way, a dual column nutritional panel will be required.
Solutions to Ensure Compliance
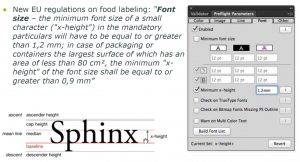
Common Preflighting Check Points:
- Fonts (min point size of positive / negative / multi color / serif / sans serif styles)
- Line weights (min point size of positive / negative / multi color styles)
- Min / Max Dot
- Small objects
- Ink coverage infringements
- Image resolutions
- Inks: max process / max spot
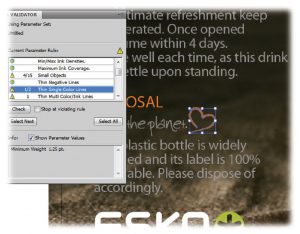
Image 5: Highlighted and zoomed in on error in Illustrator®.


